We See
Differently
Adjust each lens to fit exactly one patient.
How the Light Adjustable Lens Is Different
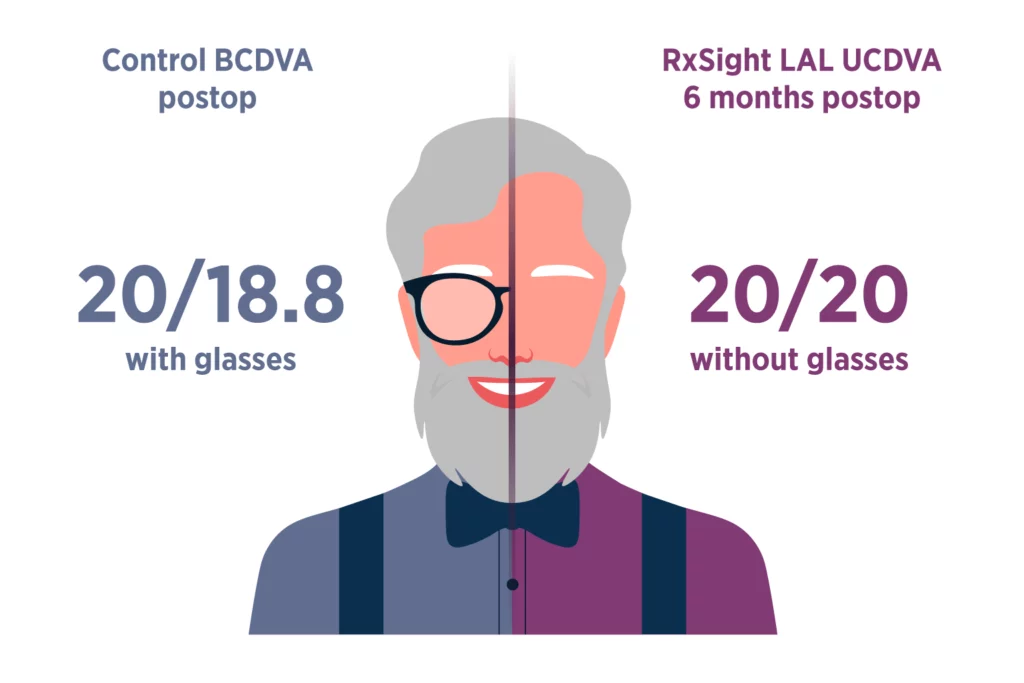
The Light Adjustable Lens has transformed cataract surgery by shifting crucial decisions regarding final lens power to the more ideal post-surgery period. This approach allows for unparalleled precision, as it accounts for lens shift and refractive changes that can occur during the healing process to achieve the best possible outcomes.
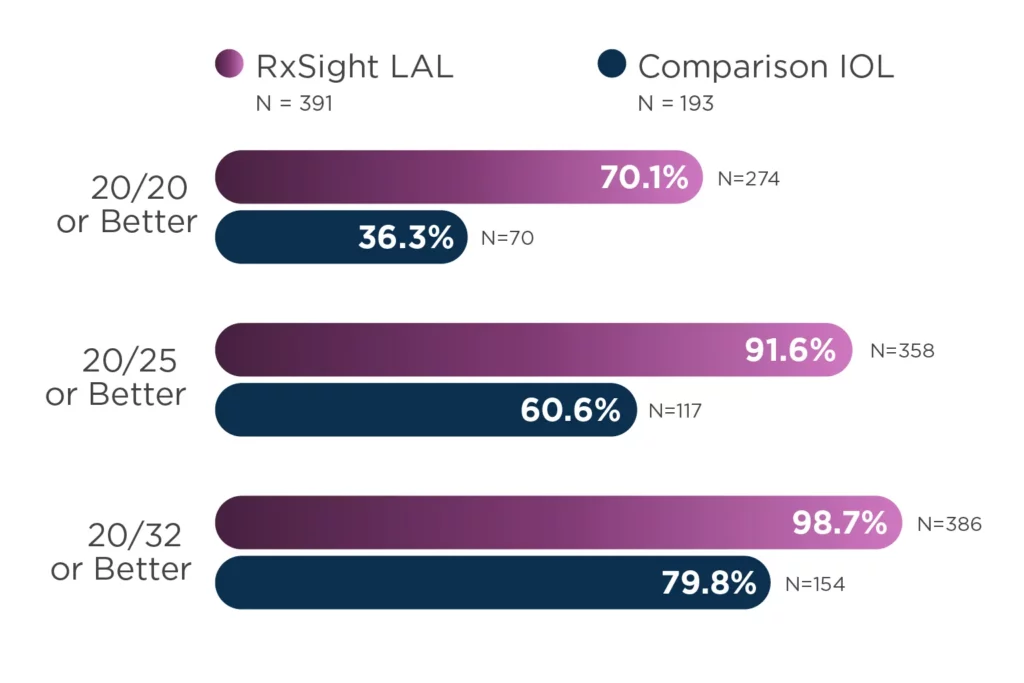
The innovative Light Adjustable Lens represents a departure from the usual practice of selecting a pre-manufactured lens power before surgery to predict a patient’s post-surgery visual outcome. The accuracy of vision achieved with the postoperative adjustability of the Light Adjustable Lens compared to traditional premium IOLs has been demonstrated in clinical studies.









Adjust for Infinite Possibilities
The Light Adjustable Lens is a versatile premium lens that a growing number of surgeons are finding is customizable for many types of patients. Throughout the adjustment process, Light Adjustable Lens patients are engaged and participate in their care in a way other lenses can’t offer.
This truly customized approach has led to outcomes they and their doctors are excited about. Almost daily, we add to the list of clinics nationwide that are successfully integrating the Light Adjustable Lens into their workflow and finding that it is a worthwhile investment for their practices and their patients.
How the Light Adjustable Lens Works
The Light Adjustable Lens is implanted using a standard cataract procedure. Patients then experience their vision and surgeons can adjust the lens over a series of treatments to ensure each patient achieves vision that matches their lives. Each adjustment is a UV light treatment performed by the Light Delivery Device™ (LDD™) that corrects refractive error and dials in optimized vision.
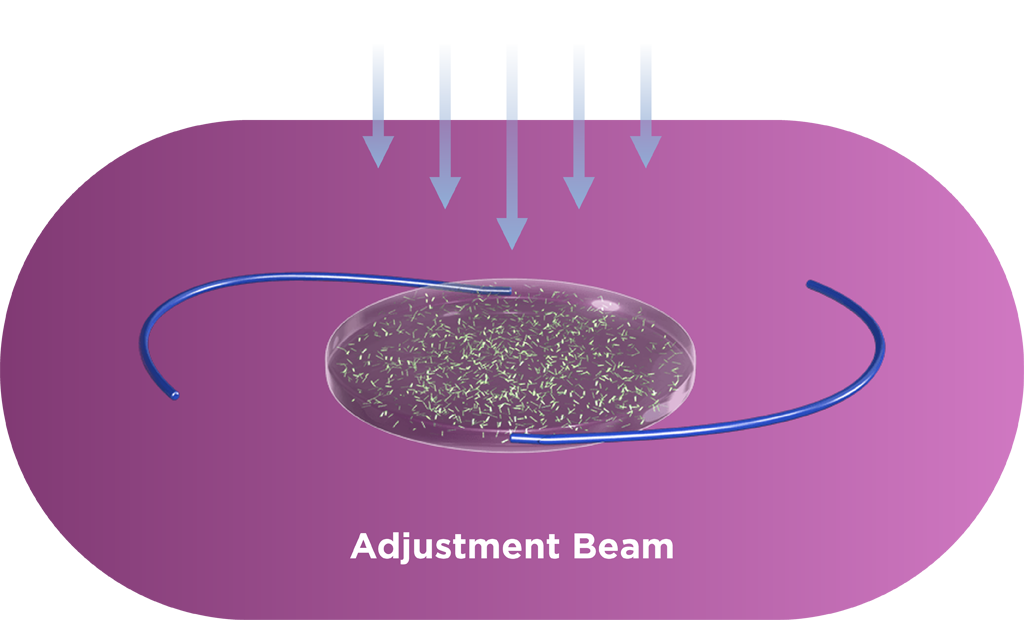
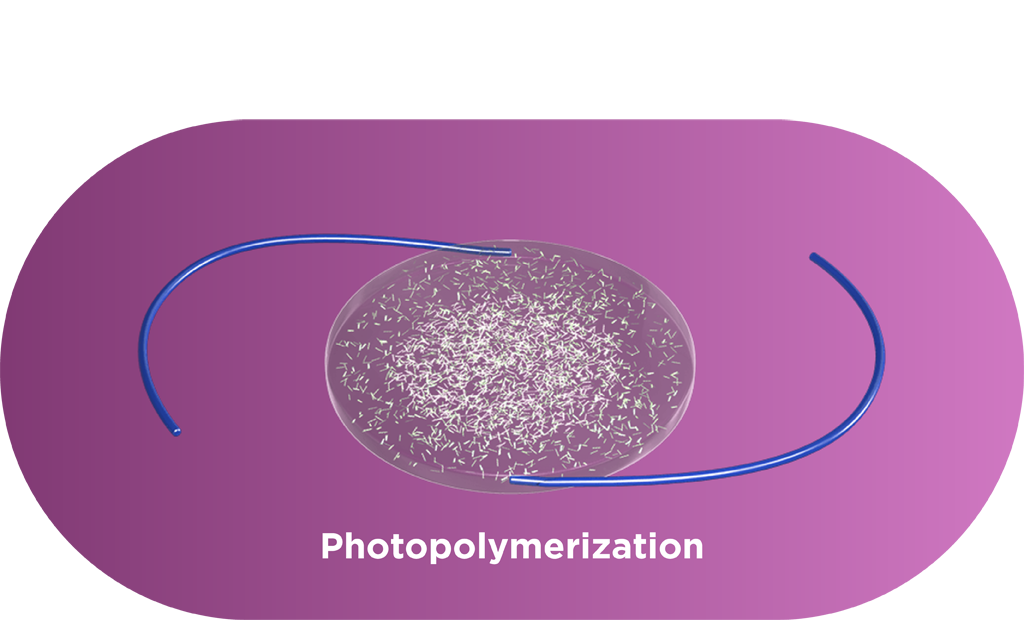

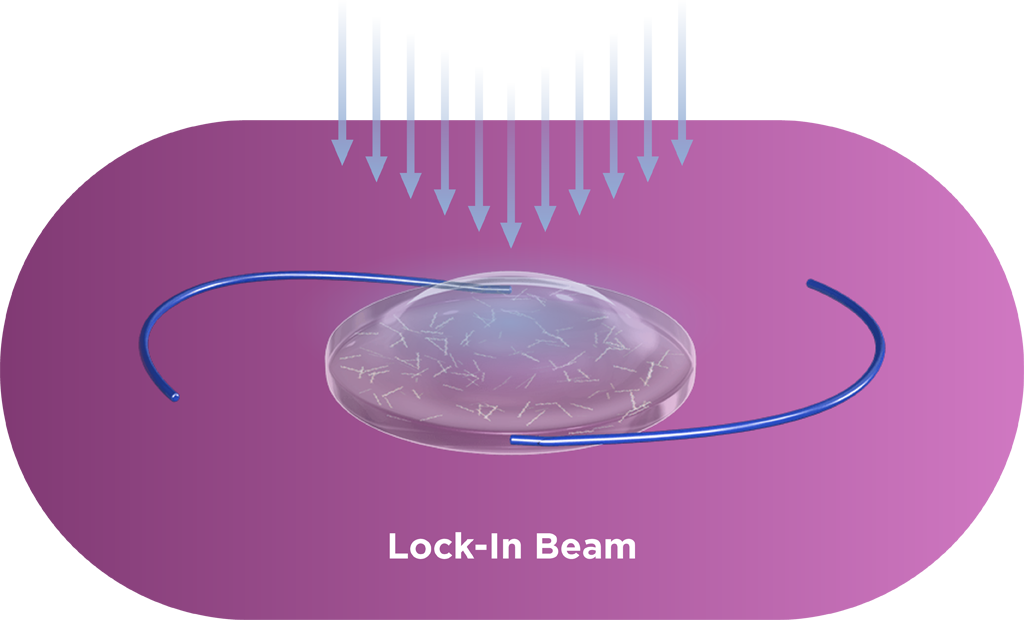
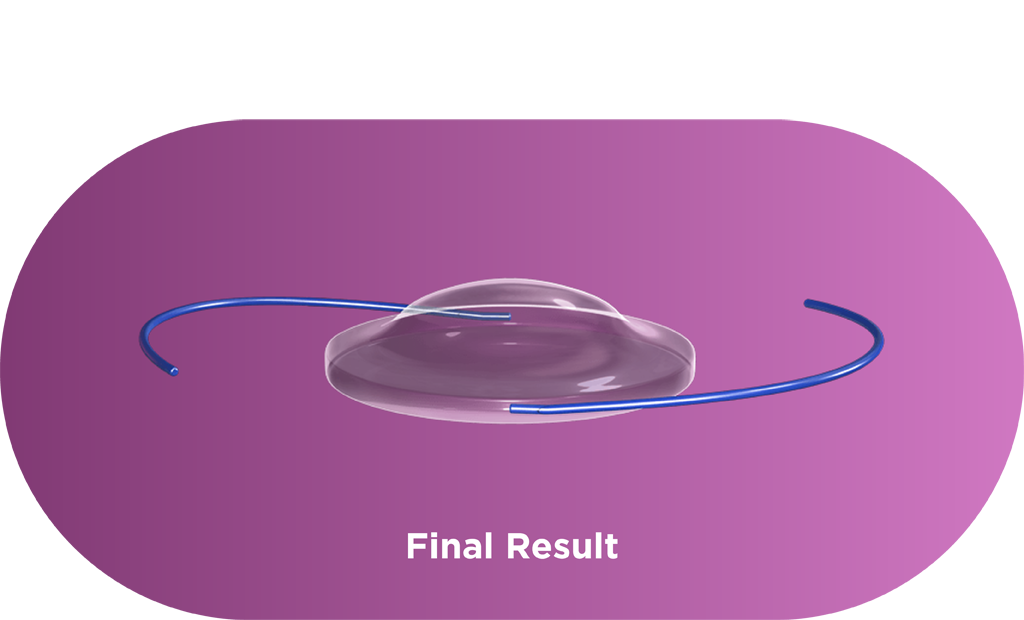
Macromers/polymers are not visible in the lens, and graphic is used only as an illustration.
The Importance of ActivShield™ Technology
ActivShield is a UV protection layer built into the Light Adjustable Lens. Along with the RxSight UV-protective glasses, ActivShield helps prevent accidental sunlight exposure from changing the lens prior to the final lock-in treatment.
- Doctor & Patient Testimonial

- Doctor Testimonial

- Patient Testimonial

- Patient Testimonial

- Patient Testimonial

- Doctor & Patient Testimonial

- Patient Testimonial
- Doctor & Patient Testimonial

- Patient Testimonial

Don’t Get Left in the Dark
Discover More About the Light Adjustable Lens
Join us in changing the shape of cataract surgery; fill out the form below to receive our complete guide to the Light Adjustable Lens.

- Watanabe K, Negishi K, Kawai M, et al. Effect of experimentally induced astigmatism on functional, conventional, and low-contrast visual acuity. J Refract Surg. 2013;29(1):19-24.
- RxSight P160055: FDA Summary of Safety and Effectiveness Data. 2017.
- Sandoval HP, Donnenfeld ED, Kohnen T, et al. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2016;42(8):1224-1234.
- 2025 RxSight Customer Survey.