RxSight® is an ophthalmic medical technology corporation headquartered in Aliso Viejo, California that has commercialized the Light Adjustable Lens™ (LAL®/LAL+™), the world’s first and only adjustable intraocular lens (IOL) that is customized after cataract surgery. The company’s mission is to revolutionize the premium cataract surgery experience by allowing surgeons to partner with their patients to achieve optimized results for every unique eye.

Dr. Kurtz has served as our President and Chief Executive Officer, as well as on our board since 2016. Prior to joining RxSight, he co-founded and served as President and Chief Executive Officer of LenSx Lasers, Inc. He became General Manager of Alcon LenSx, Inc after the company was acquired by Alcon Inc. (NYSE:ALC) in 2010. Dr. Kurtz previously co-founded IntraLase Corp. (NASDAQ:ILSE) serving as its initial President & CEO and then in other senior leadership positions. IntraLase became a publicly held NASDAQ-listed company in 2004 and was acquired by Advanced Medical Optics, Inc. (NYSE:AMO) in 2007. Dr. Kurtz has served on the faculty of both the University of California, Irvine, and the University of Michigan. He earned his B.A. in Biochemistry from Harvard College and his M.D. from the University of California, San Diego.

Mr. Weinberg has served as our Chief Commercial Officer since June 2015. Prior to joining RxSight, he was a co-founder of LenSx Lasers, Inc. and served as Chief Commercial Officer from July 2008 to August 2010, prior to its acquisition by Alcon Inc. (NYSE:ALC). He went on to serve as Vice President of Surgical Development at Alcon LenSx, Inc. from August 2010 to April 2014. He joined IntraLase Corp. (NASDAQ:ILSE) in September 1999 as Vice President of Sales and Marketing and later as the Senior Vice President, Global Marketing until the company was acquired by Advanced Medical Optics, Inc. (NYSE:EYE) in April 2007. Mr. Weinberg served as Global Director of Refractive Surgery at Chiron Vision Corp. from March 1993 until October 1997, when it was acquired by Bausch & Lomb, Inc. He continued as Global Director of Refractive Surgery at Bausch & Lomb until August 1999. Mr. Weinberg began his career in medical devices at Steinway Instruments in 1980.

Dr. Goldshleger joined RxSight, Inc. as the Vice President, Engineering and has served as our Chief Operating Officer since June 2019. Dr. Goldshleger joined RxSight in September 2015 as the Vice President of Engineering and was responsible for the development and engineering of the LAL and LDD system and its accessories. Prior to joining RxSight, Dr. Goldshleger held various management roles at Alcon LenSx, Inc. from 2010 to 2015, last serving as Director, R&D Optics and Diagnostics, and held various roles in research and development at LenSx Lasers, Inc. from October 2008 to its acquisition by Alcon, Inc. (NYSE:ALC) in August 2010. Dr. Goldshleger received a Master of Science in Physics and Mathematics from the Moscow Institute of Physics and Technology and a Ph.D. in Chemical Physics from the Russian Academy of Sciences.

Ms. Thunen joined RxSight, Inc. in January 2016 as our Chief Administrative Officer and has served as our Chief Financial Officer since February 2017. From January 2013 to October 2015, Ms. Thunen served as the Chief Financial Officer of Endologix, Inc. (NASDAQ:ELGX) From August 2010 to December 2012, Ms. Thunen served as Associate General Manager of Alcon LenSx, Inc. Prior to Alcon’s (NYSE:ALC) acquisition of LenSx, Inc. in August 2010, she served as a board member and chair of the audit committee from April 2008 to August 2010, as well as Chief Financial Officer and Vice President, Operations from November 2009 to August 2010. Ms. Thunen joined IntraLase Corp. (NASDAQ:ILSE) in May 2001 and was its Chief Financial Officer and later Executive Vice President & Chief Financial Officer until its acquisition by Advanced Medical Optics, Inc. (NYSE:EYE) in April 2007. Ms. Thunen served on the board of directors of eyeonics, Inc. from June 2007 to February 2008, and as a board member and chair of the audit committee of Restoration Robotics, Inc. (NASDAQ:HAIR) from July 2015 to November 2019, prior to its acquisition by Venus Concept Inc. (NASDAQ:VERO). She also has served as a board member and audit committee chair of Surface Ophthalmics, Inc since August 2020. She also has served as a board member and audit committee chair of Aeon Biopharma, Inc, (NYSE:AEON) and Lenz Therapeutics, Inc., (NASDAQ: LENZ) since June 2023 and November 2023, respectively. Ms. Thunen received a B.A. in economics and an M.B.A. from the University of California, Irvine.

Patrick joined RxSight as Vice President of Quality in October 2015. In February 2022, he left to serve as Vice President of Product Quality at Intuitive Surgical. One year later, he rejoined RxSight as the Senior Vice President of Quality.
Prior to RxSight, Patrick held a variety of quality management positions of increasing responsibility including Director of Quality Assurance for Alcon (Novartis) where he planned and executed projects for quality improvement, integration, and remediation of quality and compliance efforts and Director of Quality Engineering for Saint Jude Medical where his efforts led to the innovation of new products resulting in 20 invention disclosures, over 15 patent submissions, and 10 patents.
Patrick earned a Bachelor of Science degree in Mechanical Engineering from the University of Houston in Houston, Texas and a Master of Science degree in Biomedical Engineering from the University of Texas Southwestern Medical School and University of Texas at Arlington.

Debe joined RxSight in January 2016 and is Vice President of Regulatory Affairs. For the previous 30 years, Debe served as a Consultant for US and International Regulatory Affairs activities in support of investigational (clinical) and registration (marketing) applications for a wide variety of ophthalmic devices and products. Debe began her career in Regulatory Affairs at Chiron Corporation in 1993.
Debe holds a Bachelor of Science degree in Business Administration and International Management from California Polytechnic University in San Luis Obispo, California.

Jeremy joined RxSight in October 2015 and now serves as Vice President of Engineering. He started his career in the research and development of ophthalmology devices as a Mechanical Engineer at LenSx Lasers in 2009, which was acquired by Alcon in 2010. He went on to serve as Senior Mechanical Engineer at Alcon LenSx until joining RxSight.
Jeremy earned both a Bachelor of Science degree and a Master of Science degree in Biomedical Engineering from the University of California, Irvine.

With over 30 years of sales experience in ophthalmology, Steve joined RxSight in August 2021 as Vice President of US Sales. Prior to this position, he was Area Vice President, Surgical Sales Western US with Johnson & Johnson Surgical Vision. Steve began his ophthalmology sales career at Allergan Pharmaceuticals in 1989 as Senior Territory Manager and became Senior Surgical Equipment Specialist from 1999 to 2006, during which time the company was spun-off as a stand-alone company named AMO (Advanced Medical Optics). Steve held roles of increasing responsibility in refractive sales with AMO, continuing through the acquisition of AMO by Abbott in 2009. In 2014, Steve was promoted to Director of US Refractive Sales of Abbott Medical Optics prior to their acquisition by Johnson & Johnson in 2017.
Steve holds a Bachelor of Arts degree with honors in Biology from Lawrence University in Appleton, Wisconsin.

Roy is Vice President of Marketing for RxSight, having joined the company in October 2016.
In the decade prior to joining RxSight, Roy played key marketing executive roles in four successful ophthalmic medical device start-up companies. This includes Director of Marketing for AquaSys, Inc. (acquired by Allergan), Associate Director of Clinical Product Management at LenSx Lasers (acquired by Alcon), Director of Marketing Applications at Wavetec Vision Systems (acquired by Alcon), and Global Associate Product Manager at IntraLase Corp. (acquired by Advanced Medical Optics). Roy began his career in the ophthalmic medical device industry as a market research analyst at Market Scope in 2003.
Roy earned a Bachelor of Arts degree with honors in Psychology from California State University, Fullerton.

Scott joined RxSight in November 2015 as Senior Vice President of Commercial Operations & Business Process and is Certified in Production and Inventory Management. From 2010 until he joined RxSight, Scott served as Director of Technical Support & Business Systems at Alcon LenSx (Novartis), where he supported the global launch of two first-in-market ophthalmology products. He joined IntraLase Corp. in 2001, where he held a variety of logistics and planning positions of increasing responsibility until 2006, when he joined AMO (Advanced Medical Optics) as Director of Materials Management. Scott began his career at Quest Diagnostics in 1992.

Jeff joined RxSight in 2009 and currently serves as Vice President of Clinical Affairs, where he is responsible for LAL clinical research and clinical affairs. Jeff began his career as an optometrist in 2004 at EYEXAM 2000 of California and Firstsight Vision Services, where he provided primary care optometric services.
Jeff earned a Bachelor of Science degree in Biochemistry from the University of California, San Diego and a Doctor of Optometry (OD) degree from the University of California, Berkeley School of Optometry.

Matt has over 30 years of research and development experience in medical technologies, with 35 issued patents. Matt joined RxSight in May 2013 and serves as the company’s Chief Technology Officer. Previously, he was Senior Program Director at Medtronic from 2011 to 2013. Prior to this position, Matt was Vice President of Research & Development for Advanced Bionics/Boston Scientific, where he was responsible for the only US-based cochlear implant. During his 11-year tenure at Boston Scientific, Matt held roles of increasing responsibility and performed all aspects of program and engineering management. Before that, Matt was a System Engineer and R&D Manager at Acuson Corporation, an ultrasonic imaging company.
Matt earned a Bachelor of Science degree in Electrical Engineering from the University of California, San Diego and a Master of Science degree and a Ph.D. in Electrical Engineering from Stanford University.

Oliver Moravcevic assumed the role of Vice President of Investor Relations at RxSight in November 2023, bringing over a decade of experience in the medical device industry. Prior to joining RxSight, Oliver served as the Director of Investor Relations at Edwards Lifesciences, where he showcased expertise in financial modeling, shareholder engagement, and strategic communication. Preceding his role in Investor Relations, Oliver held several finance positions, including corporate FP&A, business unit finance and treasury. Before his venture into the medical device sector, Oliver accumulated valuable experience in private equity and market research at Pathway Capital Management.
Oliver holds a Bachelor of Science degree in Business Administration from the University of California, Irvine – The Paul Merage School of Business.

Maureen has over 30 years of experience in medical device regulatory affairs and clinical study management. She joined RxSight in January 2016 and serves as Senior Vice President of Clinical & Regulatory Affairs. Maureen founded O’Connell Regulatory Consultants in 1997, a consulting firm specializing in strategic planning, preparation and administration of premarket device submissions and associated clinical trials. Prior to that, she worked in executive regulatory and clinical roles for Summit Technology, Inc., a laser products company, from 1988 to 1997.
Maureen earned a Bachelor of Science degree in General Management (Finance concentration) from Boston College.

An award-winning polymer chemist, Victoria Piunova joined RxSight as Vice President of LAL Chemistry in April 2022. Victoria brings over 10 years of experience in development of materials for biotechnology, sensing, and materials discovery. Victoria earned her Ph.D. in polymer Chemistry/Materials Science from the University of Southern California and did her postdoctoral training at the California Institute of Technology in Prof. Robert Grubbs’ group, where she collaborated with RxSight on the development of UV-absorbers for intraocular lenses. Victoria continued her independent career as a research staff member at IBM Almaden Research Center, where she earned a prestigious IBM Master Inventor title and led programs on development of polymer-based drug delivery vehicles and antimicrobial polymers for application in agriculture. Victoria is a recipient of Young Investigator Award (2018) and Young Industrial Polymer Scientist Award (2021) from American Chemical Society.
Victoria earned a Bachelor of Science degree in Chemistry from Leibniz Universitat Hannover in Germany and a Master of Science degree in Photochemistry/Photobiology from Bowling Green State University in Ohio.

Chris serves as Vice President of Intraocular Lens Development at RxSight. As Founding Scientist of RxSight (formerly Calhoun Vision, Inc.) in 2000, Chris helped develop both the Light Adjustable Lens (LAL) and Light Delivery Device technologies from concept to commercial release. Chris holds 22 patents for the LAL technology and has authored numerous publications.
Chris obtained his Ph.D. from the University of Nebraska-Lincoln in 1998 and was a Post-Doctoral Scholar for Professor Julia Kornfield at the California Institute of Technology from 1998 to 2000.

Caroline is Vice President of Human Resources for RxSight, having joined the company in August 2015. Previously, she served as Human Resources Manager for LenSx Lasers starting in 2008 and continued in this position when the company was acquired by Alcon (Novartis) in 2010. She also served as Human Resources Manager from 1994 to 2001 at Total Access, a wireless communications company, and Senior Executive Assistant positions with the Swiss government and IntraLase Corp. With over 16 years of experience in the ophthalmic industry, Caroline is a passionate and experienced human resources leader with a proven history of successfully and strategically partnering with senior management to achieve organizational goals.
Caroline holds both a Bachelor of Business Administration degree in Human Resources and a Master of Business Administration (MBA) degree in Organizational Leadership from Brandman University (part of Chapman University) in Irvine, California.

Rebecca, who has held lead accounting roles in the medical device industry since 2000, joined RxSight in February 2017 as Director of Finance & Accounting. Earlier in her career, she became a licensed CPA during her tenure with Ernst & Young LLP. Since then, she has held various positions of increasing responsibility in accounting and finance with companies such as Disney, IntraLase Corp. (acquired by Advanced Medical Optics, Inc.), LenSx Lasers, Inc. (acquired by Alcon), and ReShape Medical (now ReShape Lifesciences).
Rebecca holds a Bachelor of Science degree with honors in Business Administration from Michigan Technological University in Houghton, Michigan and a CPA license (inactive) from the State of Michigan.
Approved use: The Light Adjustable Lens™ (LAL®), Light Adjustable Lens+™ (LAL+™), and Light Delivery Device™ (LDD™) system is approved for patients who have a cataract and need surgery for it, have corneal astigmatism (at least 0.75 diopters) before surgery, and do not have preexisting macular disease.
Who should not receive this treatment? The LAL/LAL+ and LDD system should not be used if you are taking medications that may increase your sensitivity to ultraviolet (UV) light; if you are taking a medication that is considered harmful to your retina; if you have a history of herpes eye infection or uncontrollable eye movements (nystagmus); or if you are unable to comply with your doctor’s schedule of LDD light treatments and instructions for wearing special UV-protective glasses for several weeks following cataract surgery.
What warnings should I be aware of? Preexisting macular disease and certain eye conditions may increase the risk of complications. Your doctor will determine if you are a good candidate for the LAL/LAL+. If you have any complications during your cataract surgery before the LAL/LAL+ is implanted, you may need to have another intraocular lens (IOL) implanted instead of the LAL/LAL+.
What precautions should I be aware of? The safety and effectiveness of the LAL and LDD have not been established in patients with certain preexisting eye conditions or in patients who experience certain complications during cataract surgery. The safety and effectiveness of the LAL+ has not been substantiated in clinical trials. The effect of the LAL+ optical design on quality of vision, contrast sensitivity, and subjective visual disturbances have not been evaluated clinically. You should discuss these issues with your doctor.
Following surgery, you must wear the special UV-protective glasses during all waking hours for about 4 to 5 weeks and comply with your doctor’s schedule of LDD light treatments. Failure to wear the UV-protective glasses can result in an unpredicted vision change or loss of vision quality after exposure to UV light, such as from sunlight. This may require a second surgery to remove the LAL/LAL+ from your eye and replace it with another IOL.
What are the potential risks? As with any surgical procedure, there are risks associated with cataract surgery and IOL implantation. Please discuss these risks with your doctor. Potential risks associated with LDD light treatments include mild alterations to color perceptions; temporary scratchiness, irritation, or dryness to the front part of your eye; and activation of a previously undiagnosed herpes eye infection. Longer lasting and serious adverse events related to the UV light exposure are possible, but rare. There is a small chance that your vision could be made worse or that you may require additional surgery as a result of a complication.
Caution: Federal law restricts this device to sale by or on the order of a physician.
©2024 RxSIGHT. All Rights Reserved. COM-1167 Rev. A
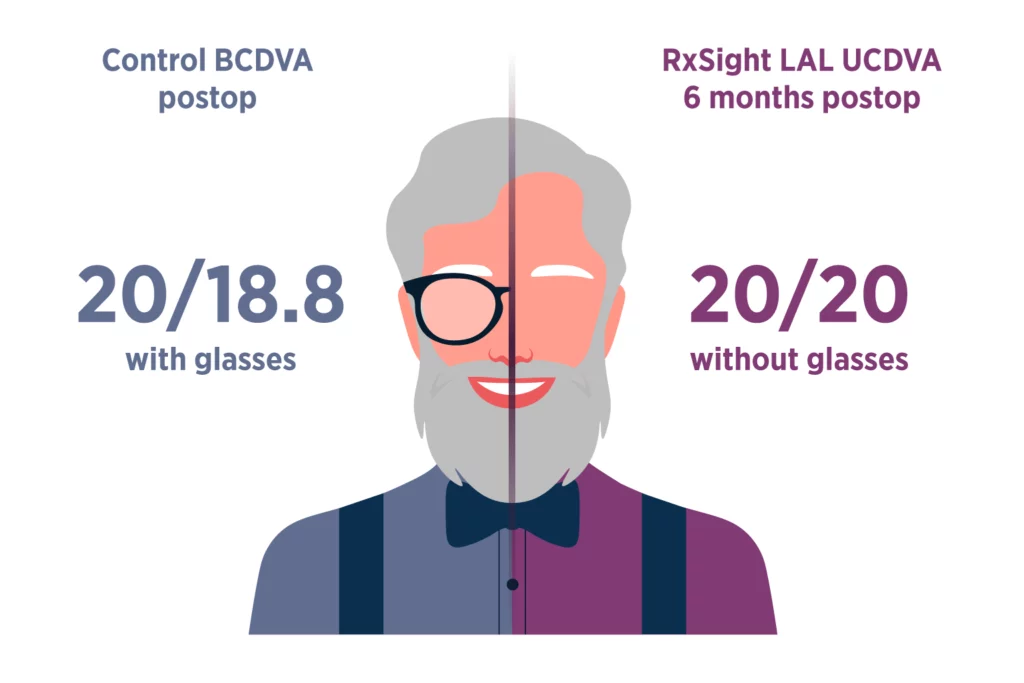
The LAL provides optimized vision for patient satisfaction.2
LAL patients saw nearly as well without glasses (UCDVA) as control patients did with glasses (BCDVA).
Since the LAL is a monofocal lens, there is low risk of dysphotopsias caused by splitting light, leading to potentially enhanced vision and patient satisfaction.
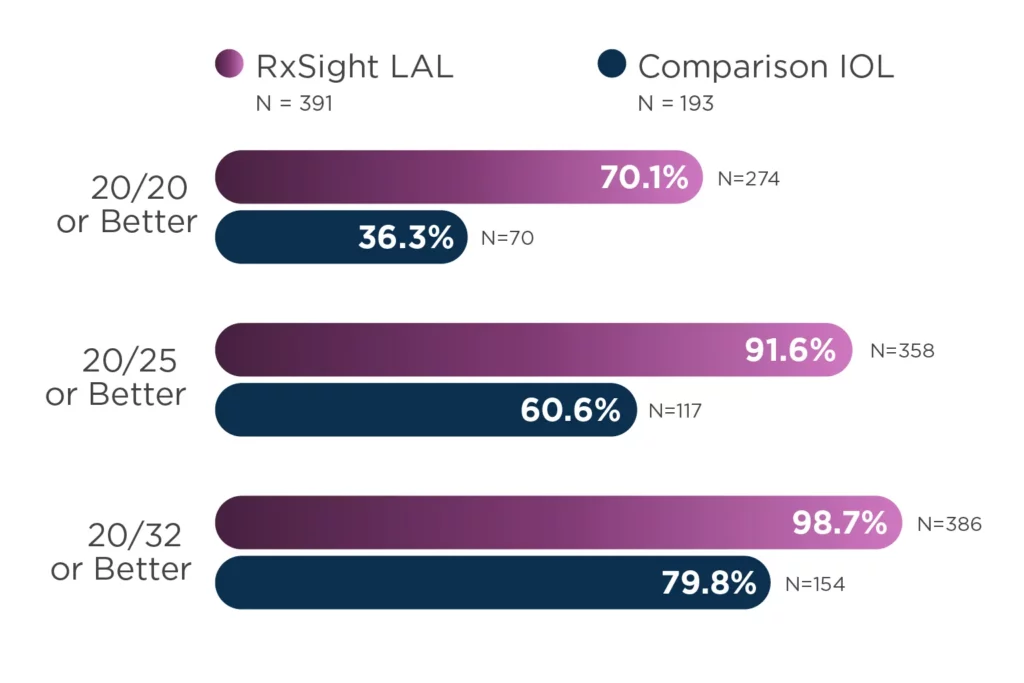
The LAL offers LASIK-like accuracy in cataract surgery.2,3
92% of eyes (N = 391) achieved results within 0.50 D of target manifest refraction spherical equivalent (MRSE).
Patients are approximately two times more likely to achieve 20/20 vision or better without glasses at 6 months.
The study was a prospective, controlled, multicenter, 12-month study of 600 patients (ITT population) randomized to receive implantation with the RxSight LAL (N = 403) or a commercially available monofocal IOL (N = 197). Effectiveness analyses included 391 LAL patients and 193 control patients. Primary safety variables included best spectacle-corrected visual acuity (BSCVA) at 6 months and incidence of sight-threatening complications and adverse events. Primary effectiveness variables included percent reduction in manifest cylinder at 6 months, percent mean absolute reduction in MRSE at 6 months, and rotation of meridian of LAL at 6 months. Percent of eyes with an uncorrected visual acuity (UCVA) of 20/20 or better at six months post-operatively compared between the LAL treatment group and the monofocal control group was a secondary endpoint.

The Light Adjustable Lens corrects down to 0.5 diopters of astigmatism, which is the lowest level approved to be treated.