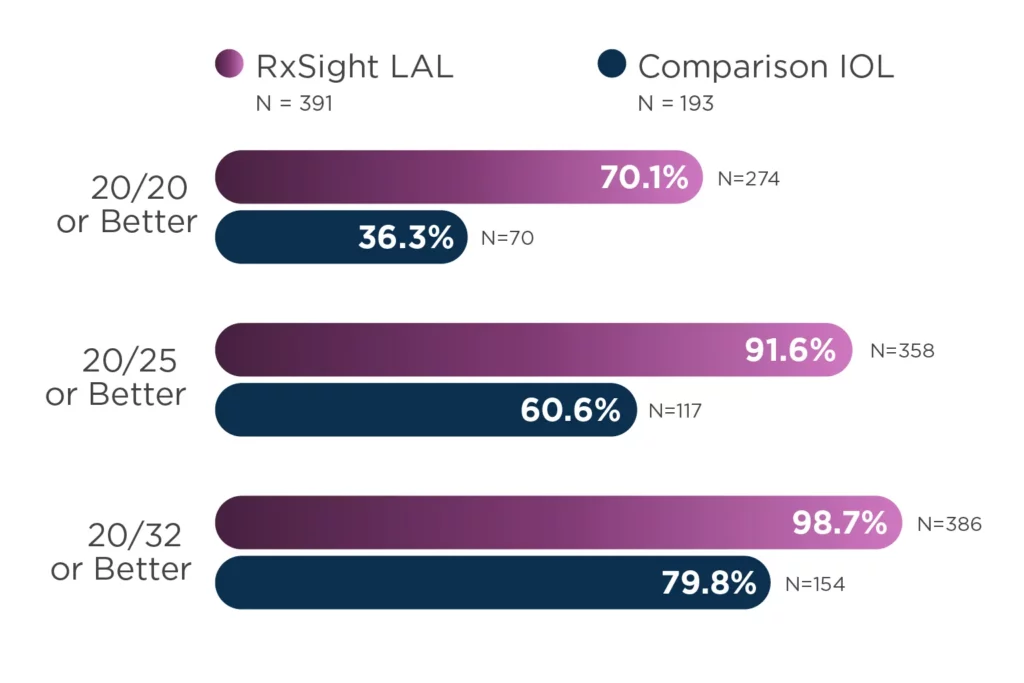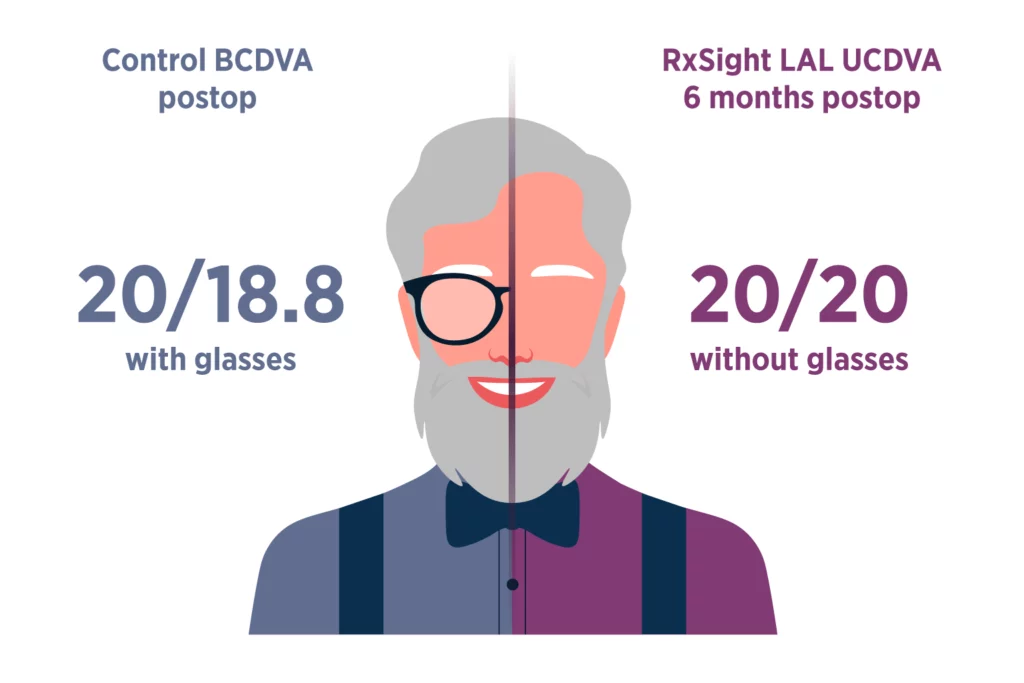
The Light Adjustable Lens provides optimized vision for patient satisfaction.2
Light Adjustable Lens patients saw nearly as well without glasses (UCDVA) as control patients did with glasses (BCDVA).
Since the Light Adjustable Lens is a monofocal lens, there is low risk of dysphotopsias caused by splitting light, leading to potentially enhanced vision and patient satisfaction.