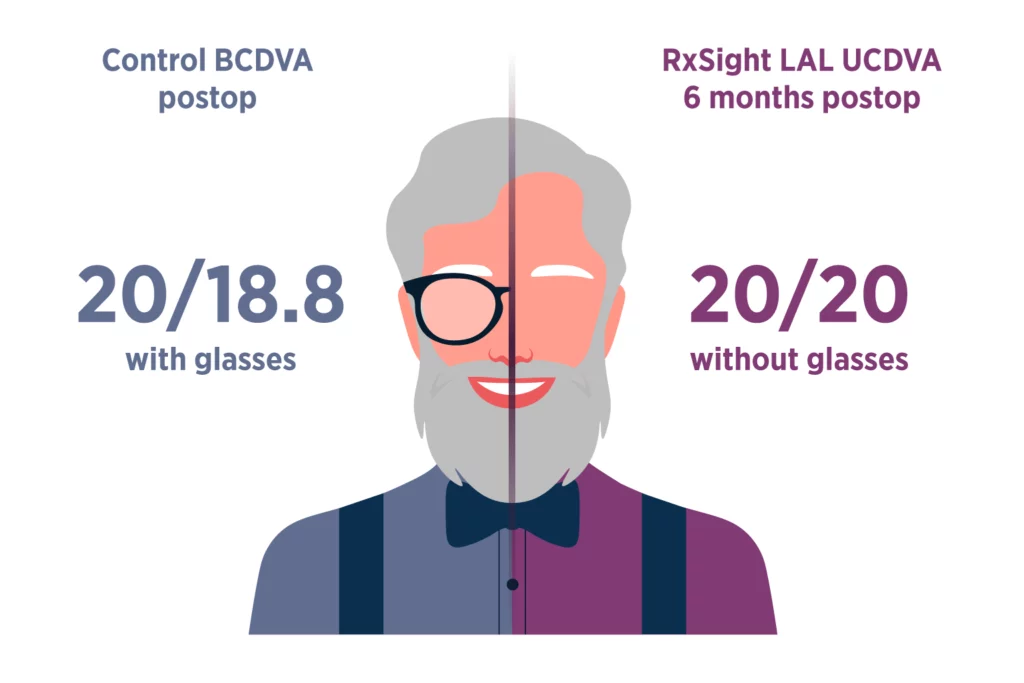
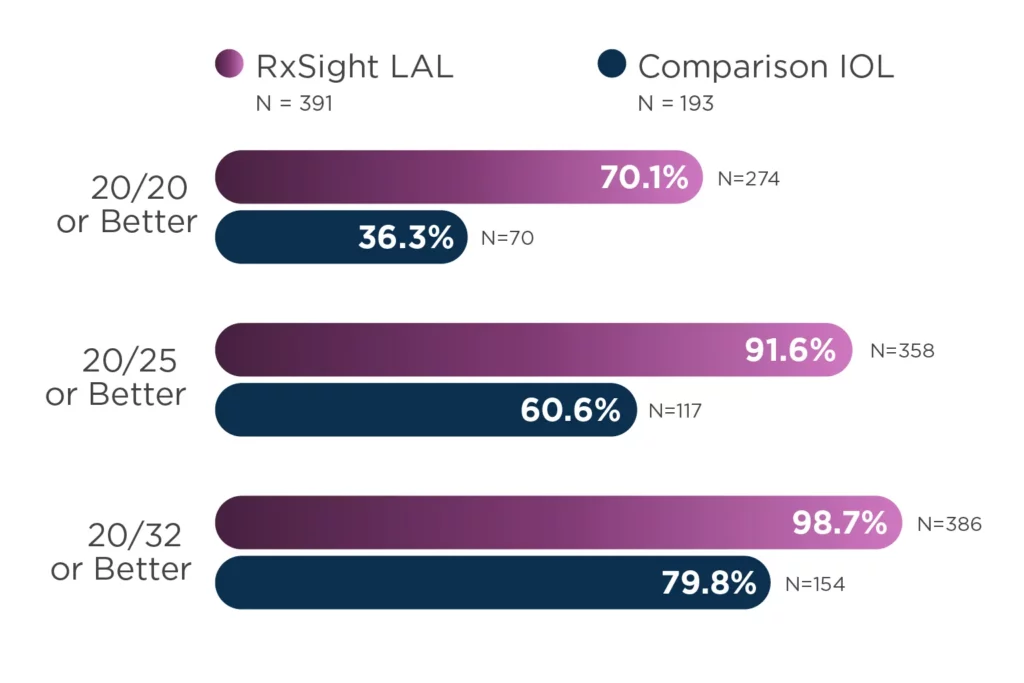
About Us
RxSight® is an ophthalmic medical technology corporation headquartered in Aliso Viejo, California that has commercialized the Light Adjustable Lens™ (LAL®), the world’s first and only adjustable intraocular lens (IOL) that is customized after cataract surgery. The company’s mission is to revolutionize the premium cataract surgery experience by allowing surgeons to partner with their patients to achieve optimized results for every unique eye.
The RxSight Executive Team

Ron Kurtz, MD, President & Chief Executive Officer
Dr. Kurtz has served as our President and Chief Executive Officer, as well as on our board since 2016. Prior to joining RxSight, he co-founded and served as President and Chief Executive Officer of LenSx Lasers, Inc. He became General Manager of Alcon LenSx, Inc after the company was acquired by Alcon Inc. (NYSE:ALC) in 2010. Dr. Kurtz previously co-founded IntraLase Corp. (NASDAQ:ILSE) serving as its initial President & CEO and then in other senior leadership positions. IntraLase became a publicly held NASDAQ-listed company in 2004 and was acquired by Advanced Medical Optics, Inc. (NYSE:AMO) in 2007. Dr. Kurtz has served on the faculty of both the University of California, Irvine, and the University of Michigan. He earned his B.A. in Biochemistry from Harvard College and his M.D. from the University of California, San Diego.

Eric Weinberg, Chief Business Development Officer
Mr. Weinberg transitioned to Chief Business Development Officer in October 2025 and previously served as our Chief Commercial Officer since June 2015. Prior to joining RxSight, he was a co-founder of LenSx Lasers, Inc. and served as Chief Commercial Officer from July 2008 to August 2010, prior to its acquisition by Alcon Inc. (NYSE:ALC). He went on to serve as Vice President of Surgical Development at Alcon LenSx, Inc. from August 2010 to April 2014. He joined IntraLase Corp. (NASDAQ:ILSE) in September 1999 as Vice President of Sales and Marketing and later as the Senior Vice President, Global Marketing until the company was acquired by Advanced Medical Optics, Inc. (NYSE:EYE) in April 2007. Mr. Weinberg served as Global Director of Refractive Surgery at Chiron Vision Corp. from March 1993 until October 1997, when it was acquired by Bausch & Lomb, Inc. He continued as Global Director of Refractive Surgery at Bausch & Lomb until August 1999. Mr. Weinberg began his career in medical devices at Steinway Instruments in 1980.

Ilya Goldshleger, PhD, Chief Operating Officer
Dr. Goldshleger joined RxSight, Inc. as the Vice President, Engineering and has served as our Chief Operating Officer since June 2019. Dr. Goldshleger joined RxSight in September 2015 as the Vice President of Engineering and was responsible for the development and engineering of the LAL and LDD system and its accessories. Prior to joining RxSight, Dr. Goldshleger held various management roles at Alcon LenSx, Inc. from 2010 to 2015, last serving as Director, R&D Optics and Diagnostics, and held various roles in research and development at LenSx Lasers, Inc. from October 2008 to its acquisition by Alcon, Inc. (NYSE:ALC) in August 2010. Dr. Goldshleger received a Master of Science in Physics and Mathematics from the Moscow Institute of Physics and Technology and a Ph.D. in Chemical Physics from the Russian Academy of Sciences.
Mark Wilterding, Chief Financial Officer
Mark joined RxSight, Inc. in January 2026 as our Chief Financial Officer. He brings over 25 years of financial leadership experience. From 2019 until he joined RxSight, Mark served as the Senior Vice President of Global Finance for Edwards Lifesciences. In that role, he was responsible for investor relations, financial planning and analysis, treasury, financial operations and strategy, and regional finance teams. He also served as Treasurer of Edwards Lifesciences from 2021 to 2024. Prior to joining Edwards Lifesciences, Mark held finance leadership roles at Medtronic plc from 2014 to 2019. Earlier in his career, he spent 15 years at Citigroup Inc. and Credit Suisse Group AG across equity research, investment banking, and capital markets. Mark received a B.A. in economics from St. Olaf College and an M.B.A. from the Kellogg School of Management at Northwestern University.

Scott Gaines, Chief Customer Officer
Scott joined RxSight in November 2015 as Senior Vice President of Commercial Operations & Business Process and as of August 1, 2025 holds the position of Chief Customer Officer. From 2010 until he joined RxSight, Scott served as Director of Technical Support & Business Systems at Alcon LenSx (Novartis), where he supported the global launch of two first-in-market ophthalmology products. He joined IntraLase Corp. in 2001, where he held a variety of logistics and planning positions of increasing responsibility until 2006, when he joined AMO (Advanced Medical Optics) as Director of Materials Management. Scott began his career at Quest Diagnostics in 1992.

Maureen O’Connell, Executive Vice President, Clinical & Regulatory Affairs
Maureen has over 30 years of experience in medical device regulatory affairs and clinical study management. She joined RxSight in January 2016 and serves as Executive Vice President of Clinical & Regulatory Affairs. Maureen founded O’Connell Regulatory Consultants in 1997, a consulting firm specializing in strategic planning, preparation and administration of premarket device submissions and associated clinical trials. Prior to that, she worked in executive regulatory and clinical roles for Summit Technology, Inc., a laser products company, from 1988 to 1997.
Maureen earned a Bachelor of Science degree in General Management (Finance concentration) from Boston College.

Patrick Cullen, Executive Vice President, Quality & Infrastructure Operations
Patrick joined RxSight as Vice President of Quality in October 2015. In February 2022, he left to serve as Vice President of Product Quality at Intuitive Surgical. One year later, he rejoined RxSight and currently serves as Executive Vice President of Quality and Infrastructure Operations.
Prior to RxSight, Patrick held a variety of quality management positions of increasing responsibility including Director of Quality Assurance for Alcon (Novartis) where he planned and executed projects for quality improvement, integration, and remediation of quality and compliance efforts. He also served as Director of Quality Engineering for Saint Jude Medical where his efforts led to the innovation of new products resulting in 20 invention disclosures, over 15 patent submissions, and 10 patents.
Patrick earned a Bachelor of Science degree in Mechanical Engineering from the University of Houston in Houston, Texas and a Master of Science degree in Biomedical Engineering from the University of Texas Southwestern Medical School and University of Texas at Arlington.

Adam Dashe, Executive Vice President, International
Adam Dashe leads RxSight’s international business strategy and global market expansion. In this role, he is responsible for driving growth across international markets, building strategic partnerships, and ensuring successful adoption of RxSight’s innovative cataract technologies worldwide. He brings more than 20 years of experience in global medical devices, with a focus on international growth and market development. Before joining RxSight, Mr. Dashe spent two decades at Edwards Lifesciences. He served as Vice President of JAPAC Business Operations for all business units beginning in 2021 and previously as Vice President for Transcatheter Heart Valves in Asia-Pacific starting in 2018. Earlier leadership roles included positions in Japan in 2015 and Europe in 2010. Earlier in his career, he managed global product portfolios, product design, and commercialization strategies for innovative cardiovascular technologies. Adam earned an MBA from the University of California, Irvine and a Bachelor’s degree in Biomedical Engineering from Washington University in St. Louis.

Jim Schindler, Senior Vice President & General Counsel
Jim Schindler joined RxSight, Inc. in October 2025 and now serves as our Senior Vice President and General Counsel. He has over 25 years of legal experience and more than 20 years in the medical device and life sciences industries. Mr. Schindler joined RxSight from Revvity, Inc. (NYSE: RVTY), a life sciences and diagnostics company, where he served as General Counsel and Vice President of Administration of BioLegend, Inc. He joined Revvity in September 2021 when it acquired BioLegend, a leader in the development and manufacture of life sciences products, where he served as its General Counsel since April 2021. Prior to BioLegend, Mr. Schindler held various legal roles at Masimo Corporation (Nasdaq: MASI), a leader in non-invasive patient monitoring technologies, from May 2011 through April 2021, ultimately serving as its Senior Vice President and Assistant General Counsel. Prior to Masimo, he served as counsel for Beckman Coulter, Inc., a medical device company, from June 2005 through May 2011. He has been a board member of Baden Sports, Inc., a sporting goods manufacturer, since June 2021. Mr. Schindler previously served as an Executive Board Director and Secretary of the Association of Corporate Counsel. He received his J.D. from the University of San Diego School of Law and holds a B.A. from Eastern Washington University. He is a member of the State Bar of California.