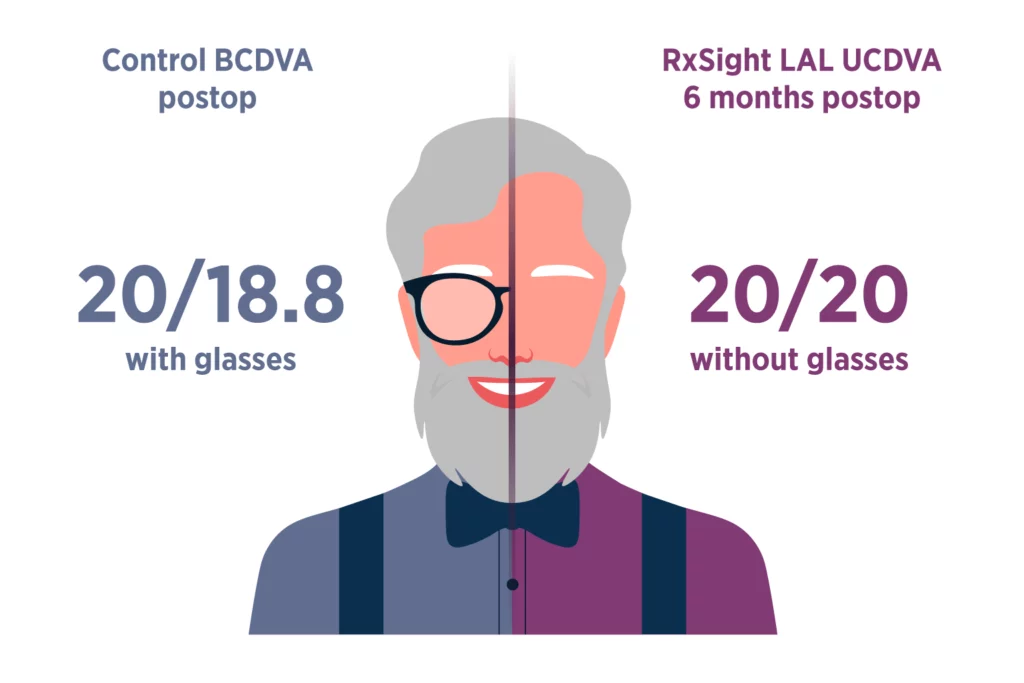
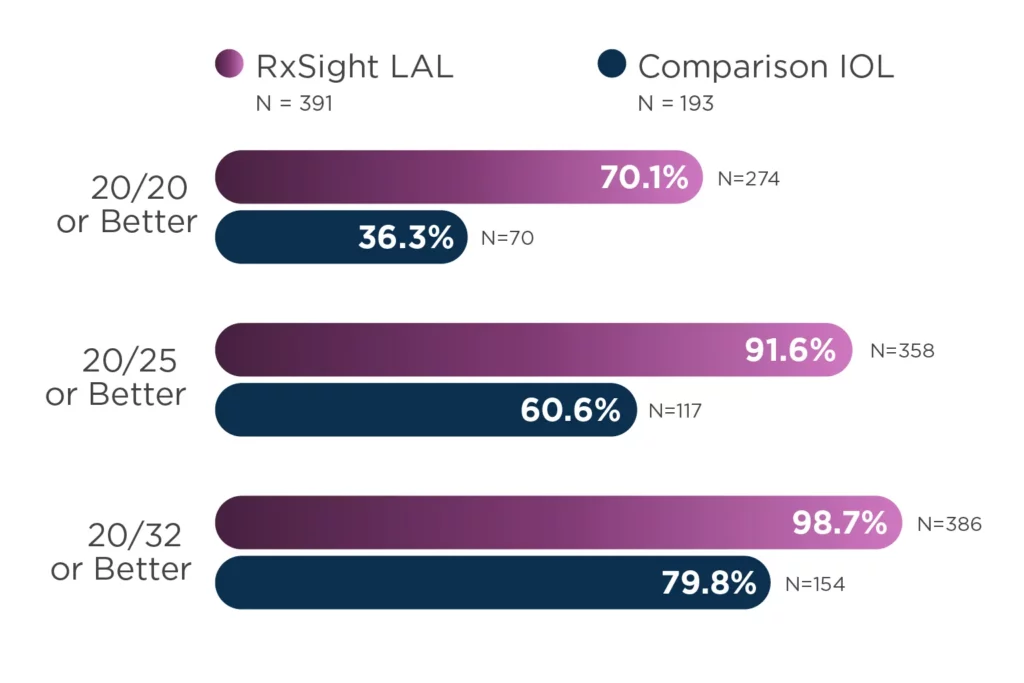
Last Updated: November 12, 2024
RxSight, Inc. (RxSight) is a medical device manufacturer that is committed to conducting its business with integrity and in compliance with all applicable laws. RxSight has established a Comprehensive Compliance Program (CCP) that is reasonably designed with consideration given to the goals of California Health and Safety Code §§ 119400 – 119402, the current operations, size, and organization of our company, and the general principles the Code of Ethics on Interactions with U.S. Health Care Professionals, a voluntary ethical code published by the Advanced Medical Technology Association (AdvaMed).
To the best of our knowledge and based on a good faith understanding of the statutory requirements in the State of California, as of the date noted below, RxSight declares that it has established a Comprehensive Compliance Program (CCP) that meets the requirements set forth in California Health & Safety Code, Sections 119400-119402. RxSight declares that, based upon its current internal tracking and monitoring systems, it is in compliance in all material respects with the provisions of its CCP.
To obtain a copy of this Declaration or an the CCP, please contact us at: compliance@rxsight.com or at 833-888-7974.